Lung cancer
In the Danish lung cancer group – DOLG – we are working on improving radiotherapy for patients with lung cancer. We have started national research projects for all stages of lung cancer.
Radiotherapy is useful for palliation of symptoms in patients with incurable lung cancer. Different dose and fractionation schedules are used in different institutions. It is unknown which treatment regime offers optimal effect and fewest side effects. PARAT is a randomized trial, that compares a short (4 days) with a long (10 days) radiotherapy regime. We will examine which of the two radiotherapy regimes that gives the fewest short term toxicity and the best symptom palliation. Quality of Life will also be examined. PARAT will include patients from all over Denmark and ensure radiotherapy at a high international level for patients with lung cancer.
- Aim: Identify the optimal choise of treatment regimen for the individual palliative lung cancer patient, taking life expectancy, predicted treatment effects, toxicity, and treatment-related impact on quality of life (QoL) into account
- Background: Radiotherapy of lung cancer struggle with low survival rates and risk of acute as well as late toxicity. The overall goal of the trials run by or with participation from DOLG is to find the best treatment for the individual patients based on i.e. stage, histology and functional imaging characteristics.
To enable this individualization for patients with curative intent DOLG is running a dose escalation trial for NSCLC (NARLAL2), participate in a dose escalation trial for SCLC (THORA) and an adjuvant immunotherapy trial for SCLC (ACHILES). Simultaneously retrospective data for patients not included in trials are being collected to develop models for patient specific treatment regimens balancing control and toxicity.
For palliative patients different RT fractionation schedules are being used throughout Denmark. The preferred fractionation scheme for patients with good PS is 30 Gy / 10 fractions (F), while patients with poor PS are offered a variety of fractionation schemes (8 Gy / 1 F, 10 Gy / 1 F, 25 Gy / 5 F, 20 Gy / 4 F, 15 Gy / 3 F) depending on institution and preference of the treating physician. Furthermore, the technical setup for palliative treatment varies across the country from simple anterior-posterior fields, with no consideration of dose to organs at risk (OAR), to advanced treatment planning using intensity modulation and image-guided set-up, minimising dose to OAR. The impact of technical treatment details and the physician-level experience with different treatment regimens is currently unknown, especially with respect to acute treatment toxicity. All major trials in this area have used simple, non-optimised techniques, with no prospective randomized studies utilizing highly conformal techniques. We expect a randomised trial with high quality RT will enable us to select the optimal RT treatment regime for the individual palliative patient.
Methods: The trial will be initiated at the Danish RT centers and are expected to begin patient inclusion before June 2018. Preparation of the trial will require improvements in delineation, planning and delivery of palliative RT and implementation is being initiated nationwide. The trial design was based on a multicenter dose-plan dummy-run. The trial is expected to include the required 1184 patients during 2 years after trial initiation.
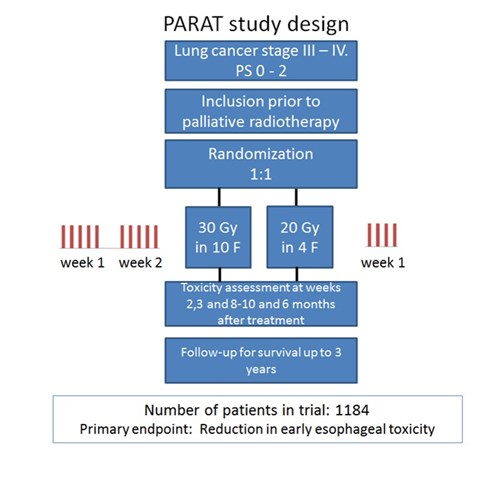
Figure 1. Study design.
As sketched in figure 1, PARAT randomized 1:1 between two different radiotherapy dose fractionations: 30 Gy/10 F or 20 Gy/4 F with:
Primary endpoint:
- Early oesophagitis (CTC AE grade 2-4, symptomatic oesophagitis with altered eating/swallowing and/or oral supplements indicated) at 2 weeks after completion of radiotherapy.
Secondary endpoints:
- Changes in symptom scores on cough, pain, dyspnoea, bronchopulmonary haemorrhage and performance status at 2 weeks, 3 weeks, 8-10 weeks and 6 months after completion of radiotherapy compared to baseline; using relevant CTC AE scales.
- Overall survival (estimated as median and 1-year survival)
- Change in global QoL at 2 weeks, 3 weeks, 8-10 weeks and 6 months post treatment compared to baseline, estimated using the EuroQol EQ-5D-5L index score.
- Disease response assessed on CT scan acquired 8-10 weeks after completion of radiotherapy
All dose plans will be exported to the national dose plan bank. Symptom scores on oesophagitis, cough, pain, dyspnoea, bronchopulmonary haemorrhage, performance status and QoL will be registered at baseline, end of treatment, 2 weeks, 3 weeks, 8-10 weeks and 6 months after radiotherapy completion. At 2 weeks and 3 weeks, symptom scores will be registered by a phone call. All pre-treatment characteristics, chosen target and RT dose, chemo- and immunotherapy regime, survival, toxicity and QoL will be registered and reported to RedCap database.
- Expected results: More than half of the lung cancer patients receive palliative RT. A better prediction of the individual expected survival and toxicity will lead to a more beneficial and individualized treatment
- Impact/Relevance/Ethics: Patients with lung cancer have a very poor survival, and it is important to improve the treatment but not at the cost of unacceptable toxicity or too much time spent at the hospital. There are no extra visits to the hospital in this trial. The patients will have to fill out two (three) short questionnaires and go through two short telephone interviews. This study will be performed according to the appropriate GCP-ICH regulations and in concordance with the Helsinki Declaration (Seoul version, October 2008). Approval from the ethical committee will be obtained before patients can be enrolled. The Danish Data Protection Agency will be notified. The patients must have received all mandatory information about the study, and are required to agree on participating by signing the consent form, before trial inclusion. The trial will be registered on ClinicalTrials.gov and clinicaltrialregister.eu.
Clinical trials
-
Rune Slot Thing
Hospitalsfysiker, ph.d.
Sygehus Lillebælt, Vejle Sygehus![]()
-
Carsten Brink
Professor, medical physics
Odense University Hospital![]()
-
Mirjana Josipovic
Hospitalsfysiker, PhD
Rigshospitalet, Copenhagen![]()
-
Patrik Sibolt
Hospitalsfysiker, PhD
Herlev Hospital![]()
-
Lone Hoffmann
PhD, clinical associate professor
Aarhus University Hospital![]()
-
Marianne Knap
Overlæge
Aarhus University Hospital![]()
-
Gitte Persson
MD, Professor
Herlev Hospital![]()
-
Ditte Sloth Møller
Head of Medical Physics, PhD
Aarhus University Hospital![]()
-
Tine Schytte
Chief physician, Associate professor, PhD
Odense University Hospital![]()
-
Wiviann Ottosson
Medical physicist, PhD
Herlev Hospital -
Marie Tvilum Chadwick Hede
PhD, MD
Aarhus University Hospital , Aarhus University![]()
-
Agon Olloni
Læge, ph.d. studerende
Odense University Hospital![]()










