Cervix cancer
Radiotherapy plays an important component in the treatment of gynaecological tumours. For most gynaecological tumours radiotherapy has been centralised to the large University hospitals in Denmark. The general aim for this research project is to improve radiotherapy through a national research strategy. All centres that are involved in the treatment of gynaecological tumours in Denmark are included in the project. The research project includes projects on radiotherapy in endometrial, cervix, vaginal and vulva cancer.
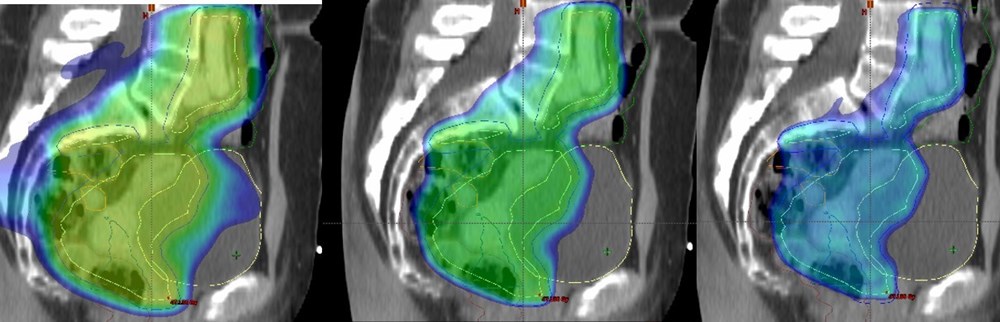
Project 1: National Danish and International multicentre study on Image-guided adaptive brachytherapy in primary vaginal cancer.
- Aims
1) Establish a benchmark for clinical outcome with image-guided adaptive brachytherapy (IGABT) in a large population with vaginal cancer.
2) Establish a reference material with regard to image-based DVH parameters for tumour and organs at risk (OAR).
3) Correlate image-based DVH parameters for tumour and OAR with outcome
- Background
During the last decade, new techniques have been developed in radiotherapy. For brachytherapy (BT) these improvements include the introduction of IGABT. This technique has shown useful in cervical cancer and resulted in improved local control rate, without increased late morbidity.
Based on these results the present study on IGABT in vaginal cancer is initiated aiming to gain evidence for the optimal treatment of vaginal cancer
- Methods
The study includes a prospective multicenter observational study (100 patients) and a subsequent interventional study (200 patients) with planning aims for tumour and OAR.
Observational study.
The dose will be prescribed in each participating center in accordance with centers general practice
Interventional study.
Based on dose-effect analysis for tumour and OAR in the observational part of the study, planning aims will be defined for the subsequent patients enrolled in the study.
- Expected results
The study will provide new information on IGABT in primary vaginal cancer: this information includes knowledge on prognostic factors for the outcome, and dose-effect for tumour and for OAR.
- Impact/Relevance/Ethics
The investigators will ensure that this study is conducted in agreement with the Declaration of Helsinki after informed consent.
Project 2: EMBRACE: Accreditation and NTCP models.
- Aims
- To achieve accreditation for enrolment of patients into the EMBRACE II study for all Danish Departments treating locally advanced cervical cancer.
- Establish NTCP models for pelvic organs based on physician-reported outcome and patient-reported outcome (PRO) in 1416 patients accrued in the EMBRACE I study.
- Background
The International study on MRI guided brachytherapy in cervix cancer (EMBRACE I) accrued 1416 patients in the period 2008-2013. In 2016, the EMBRACE II study was launched. EMBRACE II is an interventional and observational study.
- Methods
The EMBRACE II study has implemented an accreditation process including external beam radiotherapy (EBRT) and BT target definition, EBRT dose planning and prospective registration of 5 test patients. Aarhus University Hospital (AU) is already accredited for EMBRACE II. Under this project, Rigshospitalet (RH) and Odense University Hospital (OUH) will be accredited.
Statistical models for longitudinal analysis (over time) of PRO will be developed. This method will allow for the identification of patients with late and persistent morbidity. The new methodology will be applied in the EMBRACE I material and will be used to develop NTCP models.
- Expected results
All Danish cervix cancer patients will have access to the currently most advanced radiotherapy approach. High quality will be secured through accreditation and monitoring. NTCP models can be applied for the selection of patients for proton therapy.
- Impact/Relevance/Ethics
EMBRACE I and II have been/will be approved by local ethics committees.
Project 3: Vulva cancer
- Aims
- Establish a national retrospective database (2013 – 2019) with treatment-related parameters and outcome data
- Establish a prospective database with treatment-related parameters and outcome data including data on morbidity and PRO
- Background
Vulva cancer is a rare disease, with 80-100 new cases in Denmark each year. Currently, there is no prospective national registration of radiotherapy related parameters and outcome data.
- Methods
The Danish Gynaecological Cancer Group (DGCG) host a national database containing surgical data for vulvar cancer. Data regarding radiotherapy and outcome will be added to the existing national database.
The retrospective database will be separately collected and stored.
A model for PRO will be developed in collaboration with physicians treating other pelvic tumours. The PRO form will be provided electronically and the data will be kept in the existing national database. Statistical models for longitudinal analysis of PRO will be developed to allow for the identification of patients with persistent late morbidity.
- Expected results
The study will provide information on prognostic factors for the outcome, and dose-effect for tumour and for OAR, which will be beneficial for future patients.
- Impact/Relevance/Ethics
The investigators will ensure that this study is conducted in agreement with the Danish law for national database registry.
Project 4: Proton therapy in gynaecological cancer
- Aims
- To make proton therapy available to Danish postoperative cervix and endometrial cancer patients.
- Background
Proton therapy can reduce the irradiation of normal tissue in pelvic radiotherapy. In particular, there is potential to spare exposure of bowel and bladder in post-operative cervix cancer patients
- Methods
A clinical protocol will be developed which defines patient selection and proton therapy technique.
- Expected results
Danish post-operative cervix and endometrial cancer patients will have access to proton therapy.
- Impact/Relevance/Ethics
The study will be approved by local ethics committee.
Clinical trials
- EMBRACE II, ClinicalTrials.gov identifier: NCT03617133
-
Marianne Sanggaard Assenholt
Hospitalsfysiker
Aarhus University Hospital![]()
-
Kari Tanderup
Professor
Aarhus University Hospital![]()
-
Gitte-Bettina Nyvang
Overlæge (Senior Consultant)
Odense University Hospital![]()
-
Lars Ulrik Fokdal
Overlæge, Ph.d.
Sygehus Lillebælt, Vejle Sygehus![]()
-
Irene Hazell
PhD
Odense University Hospital![]()
-
Trine Jakobi Nøttrup
Overlæge
Rigshospitalet, Copenhagen![]()
-
Anders Schwartz Vittrup
Læge, PhD
Aarhus University Hospital![]()
-
Sofia Spampinato
Postdoc, PhD
Aarhus University Hospital![]()
-
Jacob Graversen Johansen
Associate Professor
Aarhus University Hospital![]()








